Adriana T. Larregina
Appointment Rank: Affiliate
Adriana T. Larregina, MD, PhD.
Dr. Adriana Larregina is a Professor with tenure of Dermatology and Immunology at the University of Pittsburgh School of Medicine and she is also a faculty member of the Graduate Program in Immunology and Microbiology (IMM).
Dr. Larregina earned her medical degree at National University of La Plata in Buenos Aires, Argentina, where she graduated Magna Cum Laude. Following, she completed a four-year medical residency in Surgical Pathology at the Center of Medical Education and Clinical Investigation (CEMIC) University of Buenos Aires, and was the chief resident during the fourth year. She then completed a fellowship in Pediatric Pathology and was an attending in Surgical Pathology.
Dr. Larregina earned her PhD in Immunology at the University of Buenos Aires School of Medicine, a degree that she obtained Summa Cum Laude. Following, she completed a postdoctoral research fellowship in Gene Therapy at the Molecular Medicine Unit, Department of Medicine of the Victoria University of Manchester (UK). Upon completion of her postdoctoral fellowship, she accepted a position of Visiting Research Instructor in the Department of Dermatology School of Medicine at the University of Pittsburgh in 1998. During the following years, she secured funding through several grants to support her research and was promoted to Assistant Professor in tenure stream, Associate Professor with Tenure, and Professor of Dermatology.
In 2003 she joined the Department of Immunology that had been recently established, and in 2009, Dr. Larregina joined the McGowan Institute for Regenerative Medicine.
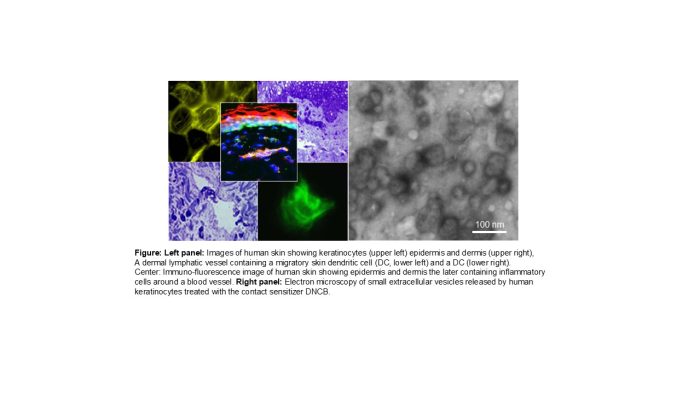
Current Research Interests
- Skin Immunology
- Role of neurogenic inflammation in the outcome of the skin immune function
- Mechanisms of cell-to-cell communication via extracellular vesicles in the skin microenvironment
My laboratory studies the modulation of the skin microenvironment by stimulation or blockade of neurogenic inflammation. We focus on how the combinatory effects of antigens and proinflammatory neuropeptides modify the T cell stimulatory function of skin resident dendritic cells (DCs) and whether blockade of neurogenic inflammation can be used to treat skin chronic inflammatory diseases. Recently, we published that preventing the pro-inflammatory function of keratinocytes by blocking the neurokinin 1 receptor, the high affinity receptor for the proinflammatory neuropeptide substance P, impairs the innate and adaptive immune responses that cause allergic contact dermatitis (ACD), demonstrating that keratinocytes are early targets and effectors of the inflammatory response initiated by skin exposure to contact sensitizers (J Allergy Clin Immunol. 150:114-130, 2022).
We also analyze the role of proinflammatory neuropeptides to induce the secretion of small extracellular vesicles by keratinocytes exposed to contact sensitizers. In fact, keratinocytes are the main targets of effector cytotoxic CD8 T cells that recognize as non-self, proteins modified by contact sensitizers and account for the chronicity and relapses of ACD. However, it is unknown how keratinocytes release contact sensitizers-modified proteins for T-cell recognition. We propose that following skin sensitization with haptens, keratinocytes release small extracellular vesicles (sEVs) containing haptenated proteins and proinflammatory mediators that modulate the activation and antigen presenting function of skin resident DCs and therefore facilitate the DC-T cell stimulatory function necessary for the development of the adaptive immune response that causes ACD.
Relevant publications
Montecalvo A, Larregina AT (First Co-author), Shufesky WJ, Beer Stolz D, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons- Weiler J, Watkins SC, and Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 119: 756-766, 2012.
Sumpter TL, Ho CH, Pleet A, Tkacheva OA, Shufesky WJ, Rojas-Canales DM, Morelli AE, and Larregina AT (Corresponding author). Autocrine hemokinin-1 functions as endogenous adjuvant for IgE-mediated mast cell inflammatory responses. J Allergy Clin Immunol. 135: 1019-1030, 2015.
Larregina AT (Corresponding author), Divito SJ and Morelli AE. Nociceptive neurons as targets to control immunity: Potential relevance for transplantation. Am J Transplant. 15: 1472-1474, 2015.
Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan ML, Gibson GA, Watkins SC, Larregina AT, and Morelli AE. Donor dendritic cell-derived exosomes promote allograft- targeting immune response. J Clin Invest. 126: 2805-2820. 2016
Li G, Larregina AT, Domsic RT, Stolz DB, Medsger TA Jr, Lafyatis R, Fuschiotti P. Skin-Resident Effector Memory CD8+CD28- T Cells Exhibit a Profibrotic Phenotype in Patients with Systemic Sclerosis. J Invest Dermatol. 2017: 137:1042-1050.
Friedrich AD, Campo VA, Cela EM, Morelli AE, Shufesky WJ, Tckacheva OA. Leoni J, Paz M, Larregina AT, and Daniel H. González Maglio. (Larregina AT last Co-autor). Oral administration of Lipoteichoic acid from Lactobacillus rhamnosus GG overcomes UVB-induced immunosuppression and impairs skin tumor growth in mice. Eur. J Immunol. 49:2095-2102. 2019
Chen Z, Larregina AT, Morelli AE. Impact of extracellular vesicles on innate immunity. Curr Opin Organ Transplant. 24:670-678. 2019.
Morelli AE, Sumpter TL, Rojas-Canales, DM, Bandyopadhyay M, Chen Z, Tkacheva O, Shufesky WJ, Wallace C, Watkins SC, Berger A, Paige CJ, Falo Jr. LD. and. Larregina, AT (Corresponding author). Neurokinin-1 receptor signaling is required for efficient Ca2+ flux in TCR-activated T cells. Cell Rep. 10:3448-3465.e8. doi: 10.1016/j.celrep.2020.02.054.PMID: 32160549. 2020
Bandyopadhyay M, Larregina AT (Corresponding author). Keratinocyte-Polyamines and Dendritic Cells: A Bad Duet for Psoriasis. Immunity. 53:16-18. 2020.
Zeng F, Chen Z, Rao C, Shufesky WJ, Bandyopadhyay M, Camirand G, Oberbarnscheidt G, Mara Sullivan M, Baty C, Yang M, Calderon M, Beer Stolz D, Geza E, Pelanda R, Todd VB, Catz SD, Watkins SC, Larregina AT (Co-Corresponding author) and Morelli AE. Graft-derived extracellular vesicles transported across subcapsular sinus macrophages elicit B-cell allo-immunity after transplantation. Sci. Transl. Med. 17 Mar 2021: Vol. 13, Issue 585, eabb0122
Bandyopadhyay M, Morelli AE, Balmert SC, Ward NL, Erdos G, Korkmaz E, Sumpter TL, Kaplan DH, Oberbarnscheidt M, Tkacheva O, Shufesky WJ, Falo Jr. LD, and Larregina AT. Skin co-delivery of hapten and neurokinin1 receptor antagonists by microneedle arrays suppresses neurogenic inflammation and contact dermatitis. J Allergy Clin Immunol. 150: 1140130 2022. Advanced on line publication Jan 29.2022.
Allen-Gondringer A, Gau D, Vargese C, Bone D, Stolz D, Larregina AT and Roy P. Vascular endothelial cell-specific disruption of the profilin1 gene leads to severe multiorgan pathology and inflammation causing mortality. PNAS Nexus. 2023 Sep 16;2(10): pgad305. doi: 10.1093/pnasnexus/pgad305. eCollection 2023 Oct.
Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks,… Larregina AT et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024 Feb;13(2):e12404. doi: 10.1002/jev2.12404.
Gaydosik A, Tabib T, Das J, Larregina AT, Lafyatis RA, Fuschiotti P.Dysfunctional KLRB1+CD8+ T-cell responses are generated in chronically inflamed systemic sclerosis skin. Ann Rheum Dis. 2025 Feb 1:S0003-4967(25)00078-0. doi: 10.1016/j.ard.2025.01.022. Online ahead of print.
Chawla P, Sharma I, Gau D, Eder I, Chen F, Yu V, Welling N, Boone D, Dr. Taboas J, Dr. Lee A, Larregina AT, Galson D, Roy P. Breast cancer cells promote osteoclast differentiation in an MRTF-dependent paracrine manner. Mol Biol Cell. 2025 Jan 1;36(1):ar8. doi: 10.1091/mbc.E24-06-0285. Epub 2024 Dec 4.
Chen R, Schneider Powell J, Shufesky WJ, Bardhi Elissa, Sullivan Mara LG, Tkacheva OA, Camirand G, Goncalves Z, Beer Stolz D, Chalasani G, Catz S, Watkins Simon C, Larregina AT (last co-author), and Morelli AE. Transplants foster B-cell alloimmunity by relaying extracellular vesicles to follicular dendritic cells. (In Press).
Powell JS, Larregina AT, Shufesky WJ, Sullivan MLG, Beer Stolz D, Gould SJ, Camirand G, Catz SD, Watkins SC, Sadovsky Y, Morelli AE. Fetoplacental extracellular vesicles deliver conceptus-derived antigens to maternal secondary lymphoid tissues for immune recognition. (In Press)
View Dr. Larregina’s complete list of publications in MyBibliography.
Funding
Current
NIH/NIAID. 1R01AI168142-01A1. Title: Role of neurokinin 1 receptor in keratinocytes in allergic contact dermatitis. Role: Principal Investigator. Years inclusive: 2022-2027.
NIH/NIAID. 1R01AI174273-01. Title: Graft extracellular vesicles as promoters of anti-donor immunity in cardiac and skin transplantation. Morelli AE (PI). Role: Co-Investigator. Years inclusive: 2022-2027.
Pending
NIH/NIAMS. Title: Role of keratinocyte small extracellular vesicles in allergic contact dermatitis.
Recently Completed
NIH/NIAID 5R01AI148690-02 (PI: Morelli). Title: Placental Extracellular Vesicles as Regulators of Maternal Adaptive Immunity. Role: Co-Investigator. Years inclusive: 7/1/2020 – 6/30/2024.
NIH/NIAMS. R01AR071277 Title: MNA Delivery of Neurokinin 1 Receptor Antagonists to Treat Atopic Dermatitis. This grant explores the possibility of manipulating the skin microenvironment to treat atopic dermatitis. Role: Co-Principal Investigator. Years inclusive: 7/1/2017 – 6/30/2022.
View Dr. Larregina’s complete list of publications in MyBibliography.